JUNE 4TH, 2020
THE FDA GRANTS IND APPROVAL TO OX2 THERAPEUTICS TO PROCEED WITH CLINICAL DEVELOPMENT OF ITS PEPTIDE LIGAND CHECKPOINT INHIBITOR FOR PATIENTS WITH CENTRAL NERVOUS SYSTEM TUMORS
OX2 Therapeutics Receives FDA Approval for Phase I Clinical Trial to Treat High-Grade Glioblastoma with Novel Peptide Checkpoint Inhibitor (CD200AR-L); First-of-Its Kind Peptide Also Being Developed As Platform for Possible Treatment of Other Solid Tumors
Immunotherapy-the Promise to Cure Cancer
“This is a fundamental change in the way that we think about cancer therapy,” said Dr. Jedd Wolchok, chief of melanoma and immunotherapeutic services at Memorial Sloan Kettering
That said:
- Researchers are challenged to overcome the complex interplay between the immune system and the immunosuppressive tumor microenvironment
- Progression to a productive immune response involves passing a complex number of immunological checkpoints that act as barriers to effective immunotherapy
- Checkpoint Inhibitors hold great promise to overcome this interplay
- Due to that promise, Checkpoint Inhibitors are and have been Fast Tracked through clinical trials by the FDA for solid tumors
- With successful funding OX2 can start a clinical trial with a high probability of gaining
this fast track annotation - CD200 is upregulated on tumor-associated vascular endothelial cells, creating an immunological barricade that inactivates T-Cells (cancer fighting cells) as they try to enter the tumor environment.
CD200 causes immune suppression in three ways:
- Tumors actively shed the CD200 protein into the circulation
- CD200 is upregulated on Tumor associated vascular endothelial cells creating an immunological barricade, inactivating T-Cells (cancer fighting cells) as they try to enter the tumor environment
- Current tumor derived vaccines actually are chocked full of CD200 which quashes the immune response
- CD200 Immune Checkpoint
- The CD200 Immune Checkpoint modulated the immune system through:
- An inhibitor receptor (CD200R1)
- Multiple activation receptors (CD200AR)
- Two in humans
- Four in mice.
CD200 CHECKPOINT PEPTIDE LIGAND Overpowers
Multiple Immune Checkpoints
OX2 Therapeutics developed a peptide ligand (CD200AR-L) that targets CD200 activation receptors (CD200AR-L), designed to be given alone subcutaneously above the draining lymph nodes
This peptide ligand activates the immune Antigen-presenting cells, resulting in:
- The CD200 Immune Checkpoint modulates the immune system through …
- CD80/86 up-regulation and production of cytokines,
- Enhances differentiation into immature dendritic cells.
- Down-regulates the inhibitory CD200R1 and PD-1/PD-L1 on Antigen-presentation cells and T cells and, in turn, inhibits the up-regulation of CTLA4
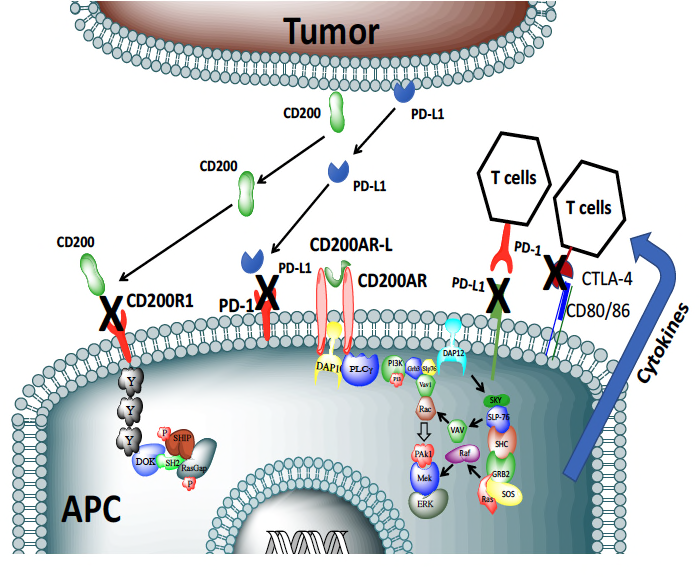
In turn, the immune system is protected from the suppressive effects of tumor-derived CD200 protein and PD-L1 in the draining lymph nodes and the tumor micro environment
This first of its kind peptide is a platform to be translatable to the treatment of other solid tumors
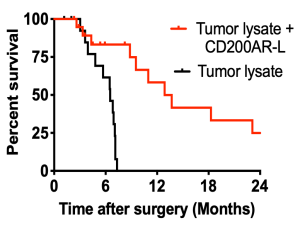
Survival Studies
We used the murine glioma cell line GL261 inoculated into the brains of mice. Mice received an autologous tumor lysate to drive a tumor specific immune response with or without the murine CD200AR-L given subdermally (Fig A).
These experiments showed a significantly increased anti-tumor response in those mice receiving CD200AR-L. We also tested CD200AR-L in a breast carcinoma model (Fig B), demonstrating the ability to translate this technology to other solid tumors. See Moertel et al., 2014.

The prognosis for dogs with glioma is extremely poor regardless of therapeutic intervention. The median survival time for dogs with glioma that do not receive any treatment ranges from 6 to 13 days and 60 to 80 days in dogs that receive surgery only. Therefore, we developed a canine-specific CD200AR-L to use in combination with autologous tumor lysate. Following surgical tumor resection, administration of intradermal CD200AR-L and tumor lysate resulted in a two-year progression-free survival of 20% (FIGURE). The peptide did not induce any systemic toxicity or other adverse effects based on serum chemistry profiles and physical exam. See Olin et al., 2019